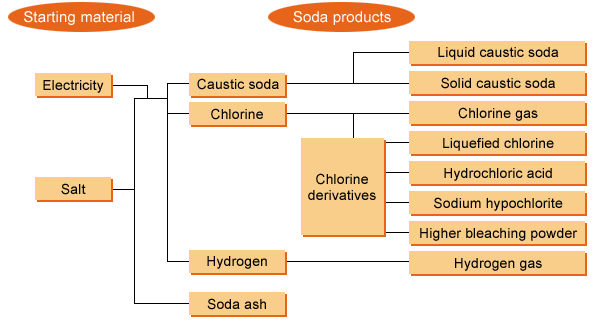
Caustic soda and chlorine
Caustic soda NaOH
Caustic soda, or sodium hydroxide (NaOH) is a well-known strong base: its aqueous solution is a strong alkali.
As such, caustic soda is used for reaction with acids (neutralization), dissolution of substances that are hard to dissolve otherwise, or reaction with metallic elements, to produce various synthetic substances or chemicals.
Examples of its utilization as basic materials include dissolution of metals, purification, removing impurities, bleaching, neutralization, and softening in various manufacturing processes of industrial and home products.
Specifically, it is essential in producing aluminum, synthetic fibers, soap and detergents, as well as dissolution and bleaching of pulp, and producing various kinds of industrial products. In addition, it is widely used for other purposes such as water treatment of water supply and sewerage, waste water from a variety of industries, and as reducing agents.
Although caustic soda is rarely an ingredient of end products, it is used to produce end products in various industrial fields, and is also used to manufacture many types of chemicals that are to become intermediate materials.
In short, in light of the utility in the various fields of industries as basic materials, caustic soda is a substance indispensable to human life.
The product is usually delivered as a 50% aqueous solution (liquid caustic soda), and sometimes in solid forms (flakes or grains) at a purity of 96% or more.
Chlorine Cl2
Caustic soda, or sodium hydroxide (NaOH) is a well-known strong base: its aqueous solution is a strong alkali.
Chlorine is one of the 100-odd elements on the earth. It is a yellowish green gas, which is heavier than air and has an irritating odor. Chlorine is a member of the halogens, along with fluorine, bromine, and iodine. It is the 11th most abundant element. Chlorine is so reactive that it combines readily with other elements, and occurs as sodium chloride (salt), potassium chloride, calcium chloride, etc in seawater and rock salt but not in the form of an elemental substance in nature.
Chlorine is generated by reacting concentrated hydrochloric acid with manganese dioxide in laboratory. On the industrial scale, most chlorine is produced by the chloralkali process.
The high reactivity of chlorine makes it useful as a reagent in many chemical processes. It is also used for disinfection and bleaching, because it has a high oxidative power and is capable of discoloring plant-derived pigments.
Chlorine as a water supply disinfectant for has a history of over 100 years.
Other important applications of chlorine include vinyl chloride resins, urethane resins, epoxy resins, synthetic rubbers, solvents, and pharmaceuticals. Chlorine thus plays an essential role in ensuring safety in human life.
Chlorine derivatives
The chloralkali process yields liquid caustic soda and gaseous chlorine at a fixed ratio. About 75% of this gaseous chlorine is supplied via pipelines to chemical plants to produce vinyl chloride, solvents, and other chemicals. The remaining 25% or so is used for manufacturing chlorine derivatives: liquid chlorine, hydrochloric acid, sodium hypochlorite, and higher bleaching powder. Chlorine is seldom marketed in gaseous form.
Liquid chlorine Cl2
Chlorine itself is marketed in liquid form (100% concentration), obtained by cooling and pressurizing gaseous chlorine, in railroad tankers, tank trucks, and cylinders.
Liquid chlorine is the most consumed among the chlorine derivatives, accounting for little less than 20% of the total consumption. The principle outlets are organic and inorganic chemicals, paper and pulp, water supply and sewerage.
Hydrochloric acid HCl
Hydrochloric acid is an aqueous solution of hydrogen chloride formed by the reaction of chlorine with hydrogen. Hydrochloric acid as an industrial product, mostly of a chlorine concentration of 35%, is classified into synthetic hydrochloric acid produced by direct reaction of chlorine and hydrogen, and byproduct hydrochloric acid formed in the manufacturing processes of chlorine-based chemicals. In fiscal year 2017, 800,000 tons of synthetic hydrochloric acid and 1.16 million tons of byproduct hydrochloric acid were produced.
Hydrochloric acid has a very wide range of applications, for example, production of heavy chemicals like inorganic chemicals, electric and electronic devices, foods, iron, and steel.
Sodium hypochlorite NaClO
This substance is produced by the absorption of chlorine by aqueous caustic soda solution, and marketed as a liquid containing 12% chlorine. There are two types of grades; the general grade contains 10-12% salt and the low-salt grade contains 4% or less.
Applications include disinfection of the water supply and sewerage and swimming pools, pulp bleaching, the food industry, water and wastewater treatment, and many others, as hydrochloric acid has a very wide range of applications.
Higher bleaching powder Ca(ClO)2
Also known as sodium hypochlorite (Ca(ClO)2) in chemical terminology, higher bleaching powder is manufactured by reacting chlorine with lime milk, and filtering and drying the crystalline reaction product. Two grade types of the industrial product are available; one contains 70% or more and the other contains 60% or more.
The principal use of higher bleaching powder is the disinfection of water, including the water supply and sewerage and swimming pools, the food industry, and wastewater treatment.
Soda ash Na2CO3
Soda ash, or sodium carbonate, is an alkaline substance of somewhat weaker reactivity than caustic soda. The lower alkalinity of the chemical is reflected in its uses, which differ from those of caustic soda.
The product, which is produced as a white powder or lumps, is classified into "light" and "heavy" grades according to density, each finding different applications. Most of the Japanese product is the heavy grade.
More than half of soda ash is used as a raw material for glass, plate glass and a variety of glass products, along with siliceous sand and limestone.
Other applications include the production of inorganic chemicals (sodium silicate, sodium bichromate, etc) and oil products.
These intermediate products are processed further into a wide range of end products, such as pigments, pharmaceuticals, detergents, adhesives, soil conditioners, leather chemicals, and plating solutions. Soda ash, which relates to various industrial activities, is thus as important as caustic soda, to manufacture various products in our modern life.
Hydrogen H2
Hydrogen, a colorless, odorless, and the lightest gas, is produced by the chloralkali process, which also yields liquid caustic soda and chlorine at a fixed ratio. A unit weight of caustic soda is accompanied by a 0.025 unit weight of hydrogen. The soda industry produced about 1.0 billion Nm3 of hydrogen in 2017.
Most of industrial hydrogen is obtained as a byproduct in oil refining, iron- and steelmaking, and petroleum chemistry. Hydrogen from the soda industry accounts for an estimated little less than 5% of the total hydrogen.
Hydrogen from the chloralkali process is suitable for the semiconductor industry because it possesses a higher purity than that from other sources; other outlets include oil and fat, glasses, metals, and chemical industries. In addition, consumption as a fuel is not negligible.
Hydrogen gas from the electrolytic cell is washed and cooled before being directly supplied via piping to other chemical processes requiring hydrogen, or compressed into cylinders for shipment after washed and cooled.